A First Look Inside Radium’s Solid-State Chemistry
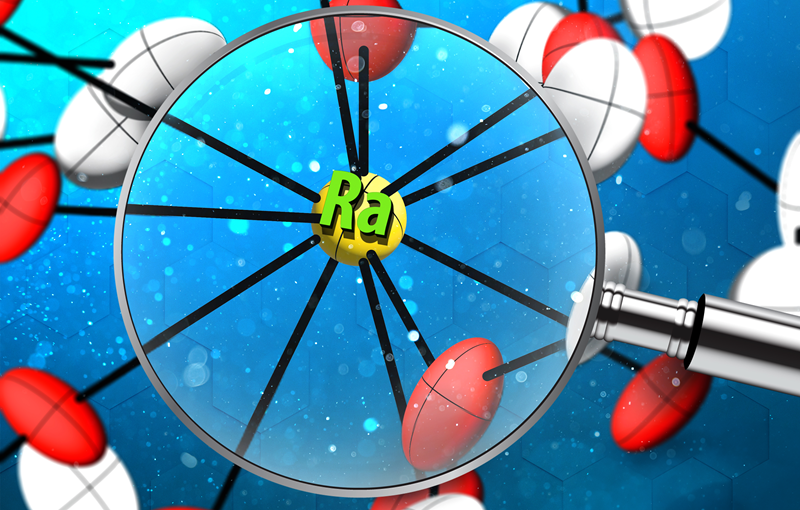
Illustration by Christopher Orosco, Oak Ridge National Laboratory Illustration of the structure of the radium compound characterized in this research. Single crystal X-ray diffraction provided detailed information on the bonding of radium in an organic molecule for the first time.
For the first time in history, scientists have measured radium’s bonding interactions with oxygen atoms in an organic molecule. Such a measurement has not been conducted until now because radium-226 is available only in small amounts and is highly radioactive (radium is one million times more radioactive than the same mass of uranium), making it challenging to work with.
The DOE Isotope Program has made a significant effort to make the rare radium-226 available for research, enabling impactful discovery research. Recently, scientists at Oak Ridge National Laboratory (ORNL), used single crystal X-ray diffraction to learn about the structure and bonding of a highly radioactive radium compound.
Working with radium required the researchers to develop a process for synthesis and crystallization on a nanogram scale. Their success using this technique to characterize radium potentially allows scientists to learn exactly how radium binds to other elements—oxygen or nitrogen, for example. Since nitrogen and oxygen are elements typically present in chelators, and radium interacts with them during bonding, this information will be helpful for developing chelators to carry radium to cancer sites in targeted alpha therapy treatment.
The methods the ORNL researchers used to characterize and analyze radium potentially could be used to learn about other challenging radioactive complexes.




