Despite being discovered less than 100 years ago, isotopes are now used in a wide variety of scientific applications that touch the lives of almost every citizen, and many of them are developed and created at NIDC production sites.
Medical Research
The development of new isotopes is critical to advancements in the medical field, ranging from new molecular imaging agents to targeted radiotherapeutics. Additionally, new production methods that provide adequate supply and reduce costs are under constant pursuit. Examples of isotopes commonly used for medical research include Fe-55, Tc-96, and Zn-65.
Diagnostic Imaging
Some isotopes emit radiation, enabling specialists to visualize the progression of disease throughout the body based on biological and physiological features. With these images, doctors can better assess how to treat the diseased tissue and also detect small cancers before they metastasize. Isotopes that aid in diagnostic imaging include Ga-68, Y-86, Sr-82, Cd-109, Fe-52, Se-72, Te-123m, and Xe-129.
Commerce, Industry, and the Environment
Numerous isotopes are used in industry and other aspects of modern life to improve productivity, enhance health and safety, and gain information that is otherwise difficult to obtain. For example, Pu-238 provides power for NASA space missions through its production of heat as it decays. Gamma radiography uses Se-75 to enable non-destructive inspection of thinner-walled steel surfaces. Certain smoke detectors contain a small amount of Am-241, whose emit alpha particles ionize surrounding air and create a current inside the chamber. Cf-252 is used for oil well logging to determine properties of geologic formations around a borehole. The radiotracer Si-32 is used by oceanographic researchers to better understand and model the global climate.
Cancer Therapy
Certain radioisotopes serve as therapeutic agents by delivering highly targeted radiation to cancerous cells while sparing side effects to normal tissues. These radioisotopes are often administered by either direct infusion or attachment to targeting vehicles, like monoclonal antibodies or peptides. Isotopes that offer cancer therapy via alpha particle emission include Ac-225, At-211, Pb-212/Bi-212, and Th-227/Ra-223; beta emitters include Cu-67, and gamma emitters include Co-60 (used in Gamma Knife® surgery).

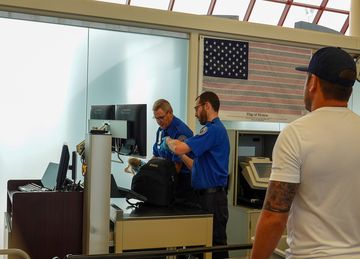
National Security
Multiple isotopes are vital to protecting the nation from physical threats. For example, neutron detectors contain lithium-6, a scintillation material, to detect the illegal transport of nuclear materials at airports, border crossings, and ports. Similarly, nickel-63 offers an inexpensive method for airport security officials to detect explosives as well as hazardous chemicals and vapors.
Nuclear Reactor Operations
Certain isotopes play a critical role in the safe and uninterrupted operation of nuclear reactors. For example, lithium-7 provides a generating reserve for the nuclear power industry to mitigate potential shortages, and uranium-234 is used to monitor neutron flux levels inside reactors.
Scientific Research
Numerous isotopes are used in various types of research. Berkelium-249, Calcium-48, and Californium-251 are all used for the discovery of new elements and in super-heavy element research.
Many other isotopes are studied for their potential as disease-fighting medical isotopes. Bismuth-213 is a decay product of uranium-233 and has promise for the treatment of certain types of cancer. Thorium-229 is widely studied for its use in the production of the medical isotopes actinium-225 and bismuth-213.