DOE Isotope Program Highlights
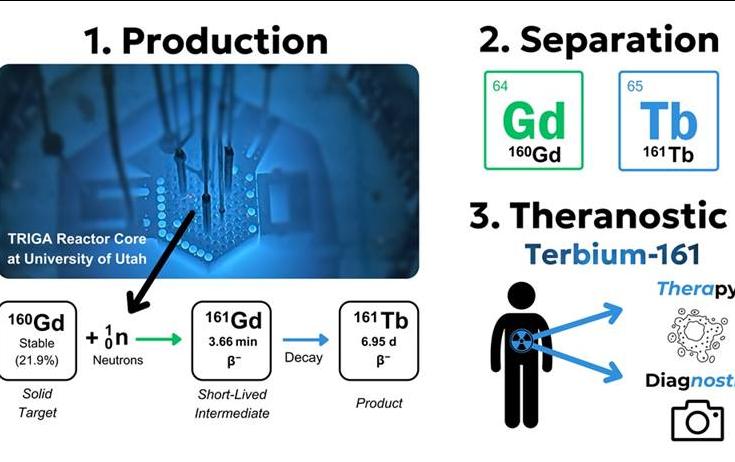
To Advance Cancer Therapy, University Starts Producing Terbium-161
In this study, researchers from the University of Utah, in collaboration with the University of Missouri, produced Tb-161 using the University of Utah’s TRIGA research reactors.

Improving Large-Scale Domestic Production of Americium-241, a Critical Component in Smoke Detectors and Nuclear Batteries
Researchers explore the effects of radiation and harsh chemicals to optimize americium-241 production.
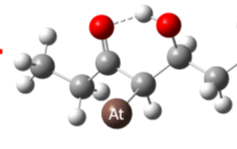
Astatine Paving the Way for a New Era in Cancer Radiopharmaceuticals
Researchers gain new insights into a strong bond between At-211 and common chemicals, creating new possibilities for cancer treatment
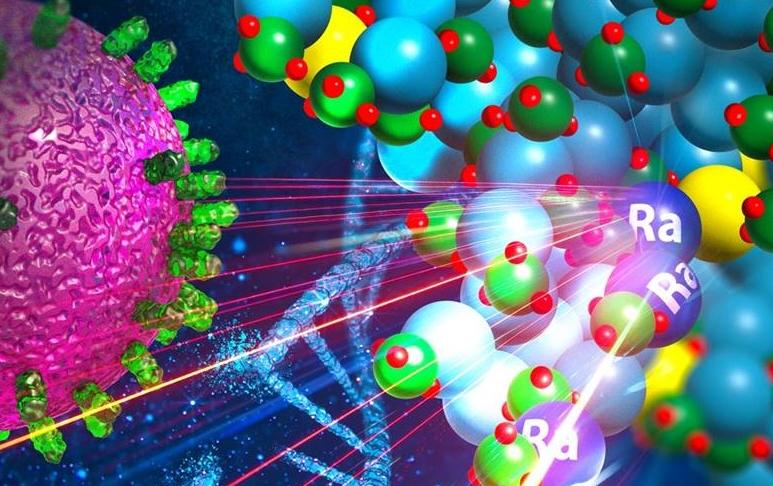
Killing Cancer with Radioactive Nanocrystals
The Department of Energy Isotope Program (DOE IP) continues to enable groundbreaking developments in cancer research through the provision of medically relevant isotopes.
Promethium Chemistry Breakthrough Could Unlock New Applications
Recently, Scientists at Oak Ridge National Laboratory were able to study the electronic structure of a promethium complex, providing new information about promethium’s chemical and physical properties.
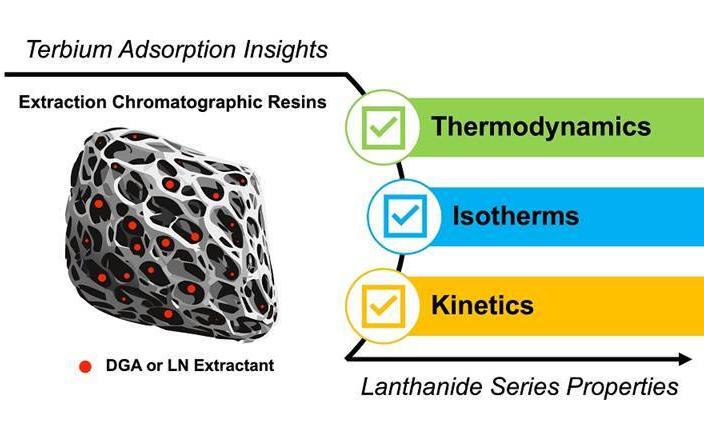
Understanding the Adsorption Properties of Terbium for Future Medical Use
In this study, supported by the Department of Energy Isotope Program, managed by the Department of Energy Office of Science for Isotope R&D and Production, researchers explored how terbium binds to these resins as a function of temperature.
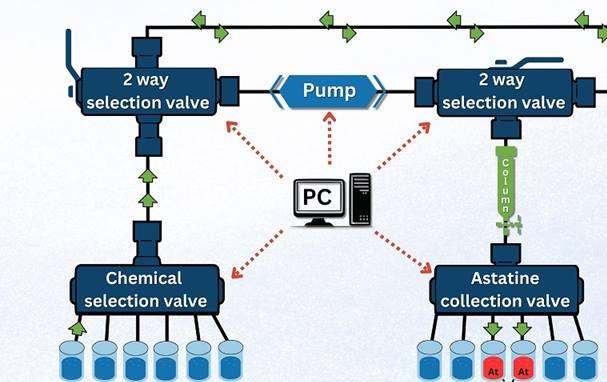
Automated Nuclear Chemistry Boosts Astatine Production for Cancer Therapy
A team of researchers designed and tested an automated protocol aimed at reducing the At-211 processing procedure from dissolution of the irradiated target through column purification in just 20 minutes.
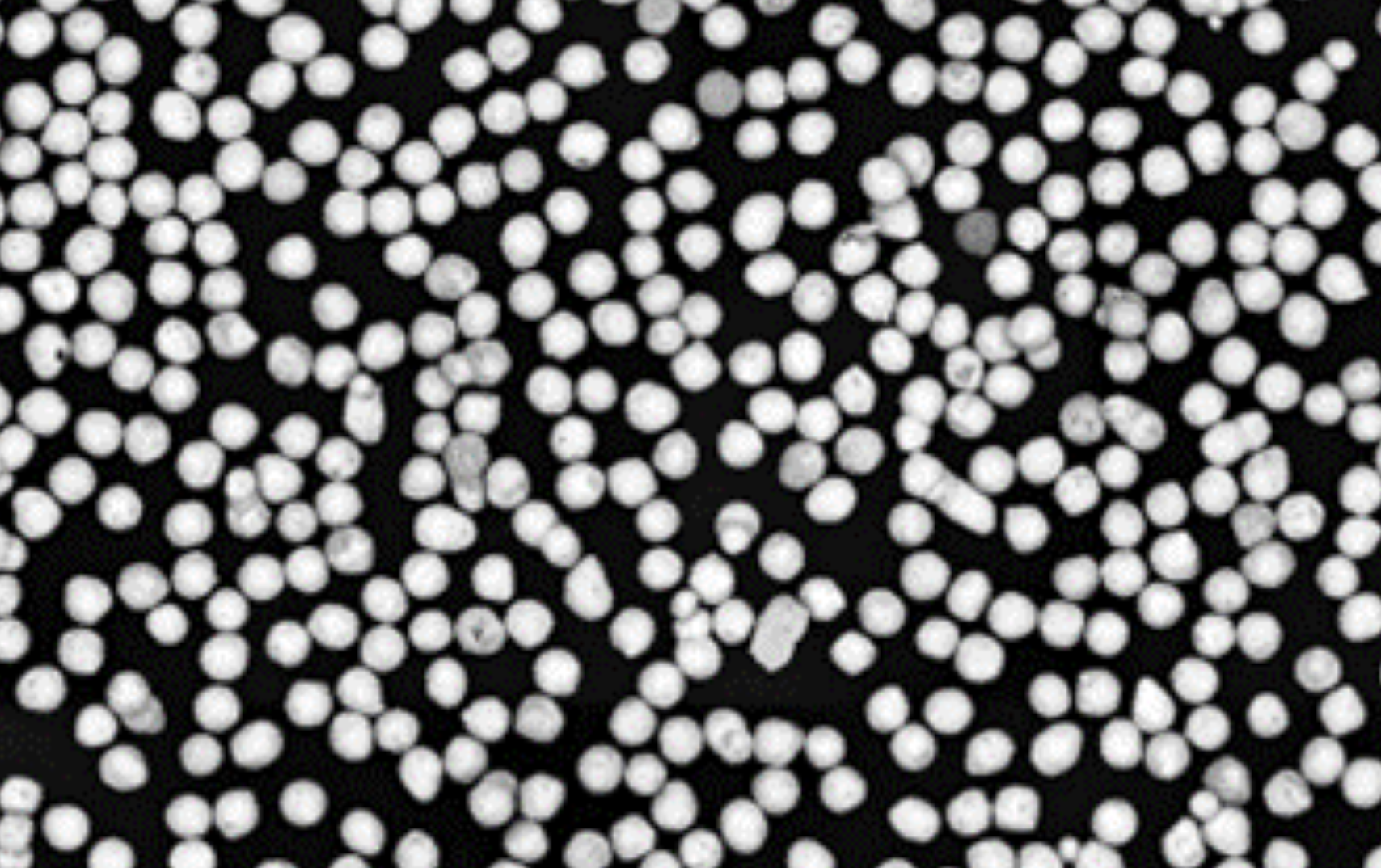
Spherical Powders Enable New Applications for Metals
Free-flowing metal powders offer improvements for additive manufacturing, isotope production target fabrication, and more.

New Understanding of Astatine’s Chemical Properties Will Aid Targeted Alpha Therapy for Cancer
Recently, scientists at Texas A&M University investigated astatine’s behavior when interacting with ion exchange and extraction chromatography resins.