Tunable Bonds: A Step Towards Targeted At-211 Cancer Therapy
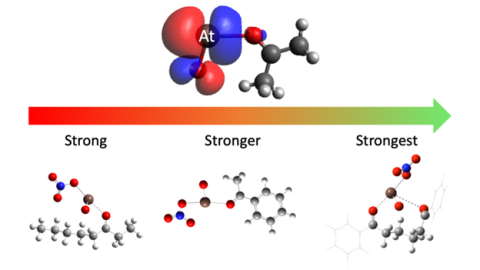
The binding of At-211 with mono- and diketones with different bond strengths. Image courtesy of Jon Burns, University of Alabama at Birmingham
Astatine is the least abundant element on Earth, and all of its known isotopes have a half-life of less than 8 hours. One astatine isotope, astatine-211 (At-211), emits alpha particles and shows promise as a cancer therapy. However, very little is known about astatine’s chemical interactions.
Researchers at Texas A&M University and the University of Alabama at Birmingham discovered a new, tunable chemical interaction of At-211 with a class of chemicals known as ketones. The interaction means scientists can tune the bond strength between ketones and At-211 by adjusting the type of ketone used. This allows scientists to control for how tightly the At-211 is held to the ketone. This discovery opens the door for developing cancer therapy drugs by linking At-211 to cancer targeting molecules.




